In alkanes the carbon is sp3 hybridized and has only 25 s-character in alkenes carbon has sp2 hybridization and hence it has 333 s-character. The alkyne does just that and a terminal alkyne is inclined to donate its protons because of a number of factors including the stability of the alkynide ion that is generated when the alkyne is deprotonated.
Pin On Alkyne Reactions With Practice Problems
Finally in alkynes the carbon is sp hybrid having 50 s-character.

Alkene vs alkyne acidity. The acidic nature is dependent on the of s-character. Amide Acid Alcohol Amine Ether Alkane Organic Functional Group Polarity and Electrostatic Potential. The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint since you can deprotonate them with a strong base like ceNaNH2 forming a carbon-based nucleophile which can then be used to make new ceC-C bonds.
Alkenes are hydrocarbons that contain one or more double bonds while alkynes contain one. Created by JayWatch the next lesson. Oxygen and nitrogen atoms have similar size.
Alkynes Alkenes Alkanes nr det gller surhet Eftersom alkyner r sp-hybridiserade och s -tecknet r 50 vilket indikerar mer verfld av s jmfrt med annan hybridisering som r sp2 och sp3 dr s -tecken r 33 respektive 25. An unsaturated hydrocarbon containing at least one carboncarbon triple bond between two carbon atoms. 0044 Deprotonation of methane0256 Deprotonation of ethane0430 Deprotonation of propane0559 Deprotonation of butane0836 Table 10850 Deprotonation of eth.
Alkenes Alkanes are the saturated hydrocarbons whereas the alkenes are the unsaturated hydrocarbons. Hybridizing the carbon so as to increase the s-character of the C-H increases the acidity with the greatest change occurring for the sp-C-H groups found in terminal alkynes. The key difference between alkenes and alkynes is that the alkenes have carbon-carbon double bonds whereas alkynes have carbon-carbon triple bonds.
A terminal alkyne one at the end of a chain is easy to spot because of the high-intensity alkynyl C-H stretch that comes at. A further difference between alkenes and alkynes is that the alkenes have no acidic hydrogen while alkynes have acidic. Electronegativity difference of oxygen and nitrogen is only 04 Pauling scale Both are very similar and differ by only number of hydrogen atoms.
Both are s p X 3 hybridized. Moreover Double bond carbons are sp 2 hybridized in alkenes and triple bond carbons are sp hybridized in alkynes. In the case of alkanes and alkenes the s character in the hybridized carbon bonds is less resulting in fewer electronegative carbon atoms and a corresponding lesser shift toward those atoms in the overlap region of the bond.
If my understanding of p K a values is correct water is nearly 10 10 times more acidic than terminal alkynes whereas ammonia is less acidic than terminal alkynes. Alkanes are undoubtedly the weakest Brnsted acids commonly encountered in organic chemistry. Terminal Alkynes as Acids A characteristic and synthetically important reaction of ethyne and terminal alkynes is salt acetylide formation with very strong bases.
Alkynes carbon-carbon triple bonds have absorptions between 2100 and 2250 cm 1 and are of medium intensity. The acidity of alkynes is greater than the acidity of alkanes and alkenes as the carbon atom in alkanes and alkenes are sp 3 and sp 2 hybridized respectively. The alkynide ion is relatively stable and donates the proton because of this stability.
Alkenes and Alkynes II Exercises. As the of s-character increases the acidic nature increases. It is difficult to measure such weak acids but estimates put the pK a of ethane at about 48.
The molecular electrostatic potential is the potential energy of. In such reactions the alkynes behaves as an acids in the sense that they give up protons to suitably strong bases. How terminal alkynes can act as weak acids and react with alkyl halides leading to their alkylation.
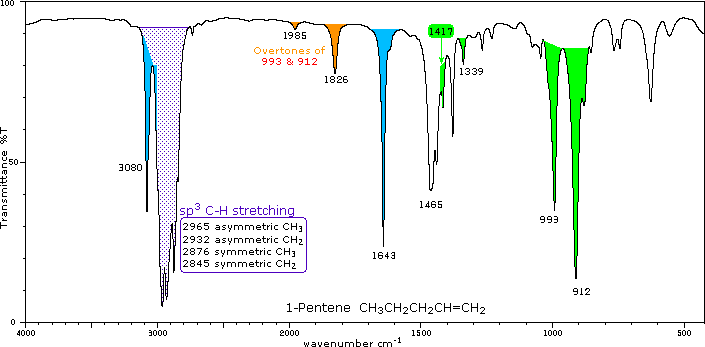
Alkanes are the hydrocarbons of containing a single bond in the main chain whereas the alkenes are the hydrocarbons of containing multiple bonds of a double bond. One of a set of the isomers of a compound that exhibits stereoisomerism. An alkene in the IR spectrum.
However the difference in acidic strength is huge. An acid is a species that donates protons. Hence these molecules contain a smaller percentage of s character in comparison to alkynes.
The location of the overlap region makes the corresponding hydrogen atoms less electron deficient and thus less acidic.
Pin On Alkyne Reactions With Practice Problems
Pin On Acids And Bases In Organic Chemistry
Pin On Organic Chemistry Notes
Alkyne Reduction To Alkene And Alkane Organic Chemistry Study Chemistry Organic Chemistry
Post a Comment for "Alkene Vs Alkyne Acidity"